The Clinical Use
- Assist subclassification and diagnosis of hematologic malignancies patients.
-
Accurately assess prognosis of these patients.
-
Identify potential targeted therapy and chemotherapy drugs options for these patients.
The Clinical Background
According to 2022 WHO system, National Comprehensive Cancer Network (NCCN), European LeukemiaNet (ELN) Guildlines and a range of authoritative research articles, a varity of types of hematologic malignancies, such as AML, MDS, MPN, lymphoma etc, the presence of certain gene variants helps with diagnosis, classification, prognosis, and therapy selection.
-
Variants in NPM1, CEBPA, and RUNX1, help define AML subtypes. Genetic testing can assist MDS diagnosis by establishing the presence of clonal hematopoiesis. EZH2, TNFRSF14, STAT6, MYD88, CXCR4, TET2, IDH1, RHOA etc gene can be useful in particular lymphoma diagnosis. The diagnosis of BMF (FA, DBA, SDS, etc) is substantially reliant on the analysis of multiple gene markers.
-
Prognostic models incorporating mutations in certain genes have been proposed to identify patients with AML, MDS, MPN as well as lymphoma to better estimate prognosis.
-
Targeted therapies are available for hematologic malignancies patients carrying mutations in IDH1, IDH2, FLT3, JAK-STAT, CCR4.
-
Pharmacogenomic testing guides precise dosing and toxicity assessment of chemotherapeutic agents in clinical practice.
The Suitable Individuals
-
Patients with suspected or newly diagnosed AML, MDS, MPN, ALL, lymphoma, and BMF.
-
Patients with relapse/refractory AML , MDS, MPN, ALL, lymphoma.
The Solutions
The AcornHema 521 analyzes genes primarily associated with hematologic malignancies, including but not limited to AML, MDS, MPN, ALL, lymphoma, BMF. More than 500 related genes were sequenced to provide necessary information diagnosis, prognostic evaluation, and treatment selection for physician.
-
CE-approved test.
-
Covers genomic regions across 521 genes.
-
Uses capture-based next-generation sequencing.
-
Supports multiple variant type detection such as single nucleotide variants (SNVs), insertions and deletions (Indels), FLT3-ITD, MLL-PTD, IKZF1 deletion, and rearrangement.
Biomarkers for diagnosis, prognosis and therapy
|
Diagnostic Classification |
Prognosis Assessment |
Therapeutic Guidance |
|
NPM1, CEBPA, RUNX1 |
KIT, FLT3, NPM1, CEBPA, IDH1/2, TP53, RUNX1, ASXL1, DNMT3A, SF3B1, U2AF1, SRSF2, ZRSR2, EZH2, BCOR, STAG2 |
FLT3, IDH1/2, NPM1, KIT |
|
Prognosis Assessment |
Therapeutic Guidance |
|
IKZF1, TP53, NOTCH1, FBXW7 |
ABL1, JAK3, JAK1 |
|
Diagnostic Classification |
Prognosis Assessment |
Therapeutic Guidance |
|
JAK2, CALR, MPL, CSF3R, ASXL1, EZH2, TET2, IDH1/2, SRSF2, SF3B1 |
JAK2, CALR, MPL, ASXL1, EZH2, IDH1/2, SRSF2, TP53, SH2B3, SF3B1, U2AF1, ABL1 |
JAK2, CSF3R, ABL1 |
|
Diagnostic Classification |
Prognosis Assessment |
Therapeutic Guidance |
|
SF3B1, TET2, ASXL1, DNMT3A, SRSF2, RUNX1, TP53, U2AF1, EZH2, ZRSR2, STAG2, CBL, NRAS, JAK2, SETBP1, IDH1/2, ETV6 |
ASXL1, EZH2, SF3B1, SRSF2, U2AF1, ZRSR2, RUNX1, TP53, STAG2, NRAS, ETV6, SETBP1, BCOR, FLT3, WT1, STAT3 |
TET2, STAT3, TP53 |
|
Diagnostic Classification |
Prognosis Assessment |
Therapeutic Guidance |
|
TET2, SRSF2, ASXL1, RUNX1, NRAS, CBL, SETBP1, ETNK1, PTPN11, NF1, KRAS, JAK2, JAK3, SF3B1, MPL, CALR |
ASXL1, EZH2, SRSF2, SETBP1, BCOR, JAK3 |
JAK2 |
|
Diagnostic Classification |
Prognosis Assessment |
Therapeutic Guidance |
|
TP53, MYD88, BRAF, MAPK1, CXCR4, CF3, ID3, BCL6, MAP2K1, NOTCH2, KMT2D, KLF2, SPEN |
TP53, SF3B1, ATM, BIRC3, NOTCH1/2, KLF2, BCL6 |
TP53, BTK, PLCG2, NOTCH1, SF3B1, BIRC3, MYD88, CX CR4, BCL6, BCL2 |
|
Diagnostic Classification |
Prognosis Assessment |
|
STAT3, STAT5B, ATM, JAK1 |
STAT5B |
|
Diagnostic Classification |
|
CEBPA, DDX41, ATG2B, GSKIP, ANKRD26, ETV6, GATA2, RUNX1, SAMD9, SAMD9L, SRP72 |
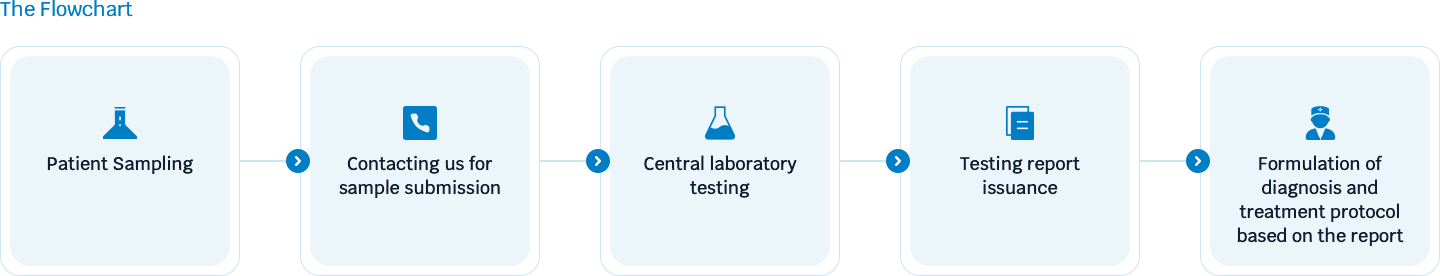
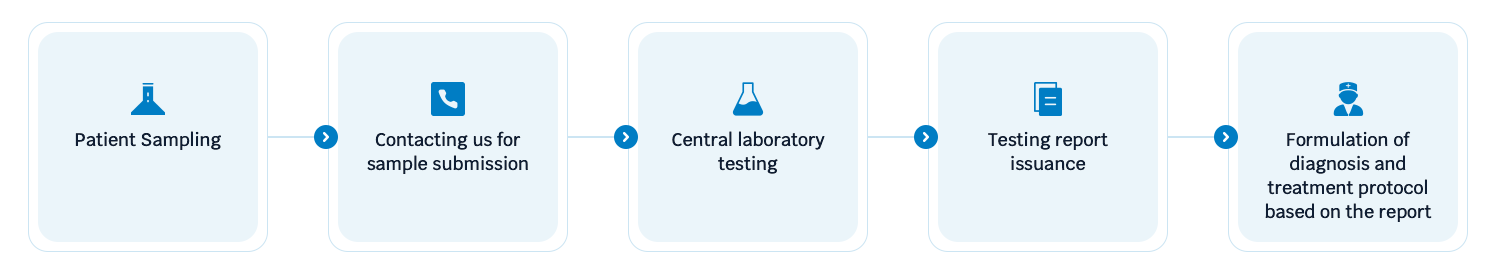
Flowchart
 Urothelial Carcinoma Products
Urothelial Carcinoma Products Prostate Cancer Product
Prostate Cancer Product Solid Tumor Product
Solid Tumor Product Hematologic Malignancy Product
Hematologic Malignancy Product
