The Need
Bladder cancer (BC) is the 5th most common cancer in the western world, and 70%-80% of the patients are diagnosed with non-muscle invasive disease (NMIBC). BC has a high risk of recurrence, around 50% of NMIBC patients will experience a recurrence. As a result of the risk of recurrence and progression, patients with NMIBC need surveillance following therapy. Follow-up should be repeated every 3 months for a period of 2 years, and every 6 months thereafter until 5 years, and then yearly.
Follow-ups methods include cystoscopy procedures, Urine cytology and Urinary molecular marker tests.
- Cystoscopy: Cystoscopy enables the inside of the urethra and bladder to be examined and sampled. Cystoscopy procedures that are invasive and painful, are negative in 90% of cases, and detect only 70-80% of recurrences.
- Urine cytology: This test involves examining a urine sample under a microscope to determine if cancer or pre-cancer cells are visible. The Urine cytology has high sensitivity in HG and G3 tumours (84%), but low sensitivity in LG/G1 tumours (16%).
- Urinary molecular marker tests: Research has been carried out into the usefulness of urinary molecular markers in the follow-up of BC. The most commonly used methods are FISH and NMP22. Urine FISH has a sensitivity of 63% overall and 41% for low-grade cases, while NMP22 has a sensitivity of 58% overall and 25% for low-grade cases. Additionally, clinical factors like bladder inflammation and calculi affected Fish and NMP22.
The Solution
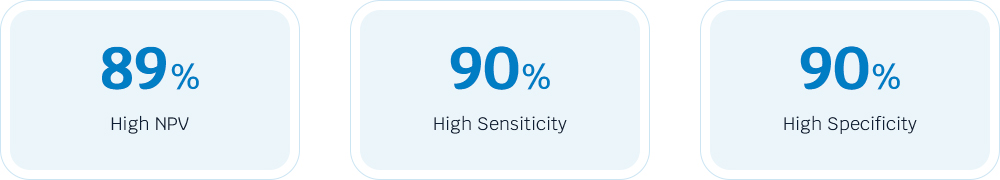
AcornUI-MRD provides patients and clinicians with a simple, objective urine test to detect recurrence of BC. It demonstrates high NPV, meaning that when receiving a negative result, may help patients avoid unnecessary procedures and discomfort. AcornUI-MRD is a highly sensitivity and specificity, which ensures more accurately assess the risk of recurrence or the presence of bladder cancer.
AcornUI-MRD can be used in a surveillance regimen to increase confidence in detection of recurrence and/or to reduce amount of cystoscopies.
- The accuracy of AcornUI-MRD was validated in a clinical trial.
- AcornUI-MRD is an in vitro diagnostic device for the detection of DNA methylation patterns in urine that are associated with BC.
- Quantitative Real-time PCR (qPCR) method makes the test economically and quickly.
- The non-invasive and painless test uses urine samples.
- Sample stability for up to 7 days in AcornUI-MRD Urine Transport Reagent.
- Turn-around time: 1-4 days from receipt of specimen.
The Evidence
National Comprehensive Cancer Network (NCCN) Guidance recommends that evaluation of urinary urothelial tumor markers may be considered during surveillance of high-risk NMIBC.
European Association of Urology (EAU) Guidelines recommends Non-invasive follow-up strategies include urinary molecular marker tests as adjunct (or companion) tests to improve detection at the time of cystoscopy or as replacement tests to reduce the number of cystoscopies.
The AcornUI-MRD Validation Data enrolled 1188 participants, the data from this trial shows that this test is capable of accurately predicting BC recurrence in the urveillance phase.
- The AcornUI-MRD demonstrates high NPV of 89%, and is a highly sensitivity and specificity both of 90%.
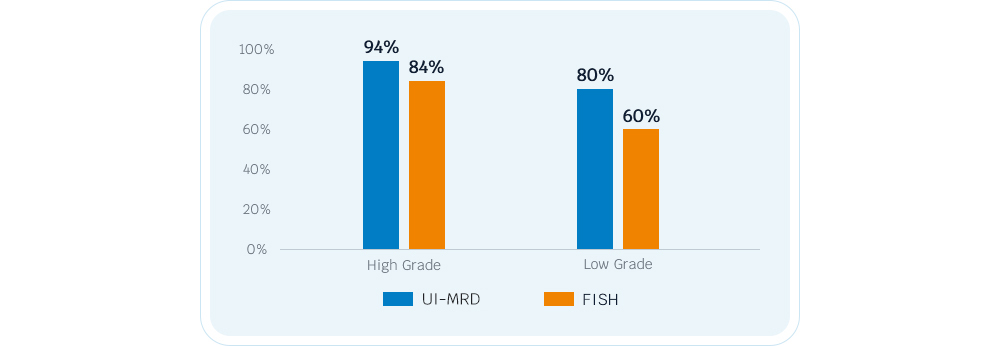
- The AcornUI-MRD has extremely high sensitivity, making it suitable for detecting both high-grade and low-grade BC compared to FISH. It can be used in follow-up to replace or postpone cystoscopy.
The Suitable Individuals
- In suitable cases post TURBT, can alternate with cystoscopy to de-intensify surveillance.
- May be used to replace cystoscopy in patients with no recurrence long term (low risk 3+ years, high risk 5+ years) after shared decision making with the patient.
 Urothelial Carcinoma Products
Urothelial Carcinoma Products Prostate Cancer Product
Prostate Cancer Product Solid Tumor Product
Solid Tumor Product Hematologic Malignancy Product
Hematologic Malignancy Product