The Need
Prostate cancer (PCa) is the most frequently diagnosed malignancy among men worldwide, with annual treatment expenditures approaching US $12 billion. The incidence of PCa is closely related to age, with an exponential increase over the age of 50. Each year, around 500,000 biopsies are conducted on men, a significant number of which may not be needed.
The common methods include PSA testing, DRE, imaging examinations and biopsy.
- PSA: PSA is not a cancer-specific marker, and as such most individuals with elevated PSA levels do not have PCa. Fewer than 25% of men with elevated PSA would have a type of PCa which needs immediate treatment. It has led to over-treatment of indolent clinically insignificant prostate cancer and performance of large numbers of negative biopsies.
- DRE: DRE is highly dependent on the clinical experience of the examiner. DRE should not be used as a stand-alone method for the early detection of prostate cancer, because DRE alone misses a substantial number of clinically significant cancers.
- Imaging examinations : The examinations results are often easily influenced by experience of the physician. The positive rate of PCa on MRI was only 35% in patients with suspected PCa.
- Prostate biopsy: It needs taking 10-12 core samples then performing histopathological examination. It is the gold standard for diagnosing prostate cancer. However, about 40% of prostate biopsies can be associated with discomfort andcomplications, such as bleeding, infection, urinary incontinence, and sexual dysfunction. It is established that 70-80% of prostate biopsies show no cancer or clinically insignifcant slow gowing cancers.
- In recent years molecular marker tests have been emergingis coming up. As a accuracy screening method it has high sensitivity and NPV to assist doctor diagnosing or ruling out PCa avoiding unnecessary.
The Solution
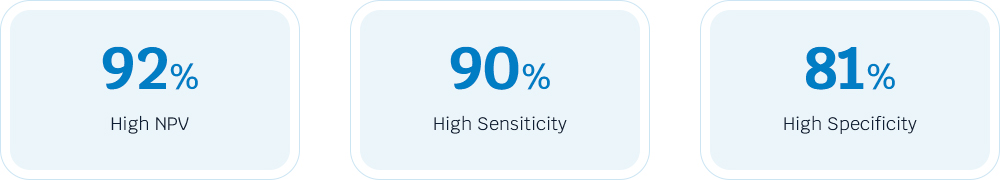
The AcornUPro-SEEK is a simple, non-DRE, and innovative urine test to detect PCa. It exhibits high sensitivity, facilitating accurate diagnosis of PCa , effectively for clinically significant prostate cancer. Additionally, high specificity can rule out PCa and avoid unnecessary biopsies. Especially with a negative predictive value (NPV) of 92%, if the test identifies a very low risk, the physician can be confident that there is a 92% likelihood that the patient does not have GS≥7 PCa and can therefore avoid a biopsy.
-
The accuracy of AcornUPro-SEEK test was validated in a clinical trial.
-
The Multiomics-based molecular biomarker test were intensively validated for the detection of PCa, and algorithm independent of PSA and standard clinical parameters.
-
Quantitative Real-time PCR (qPCR) method makes the test economically and quickly.
-
The non-invasive and painless test uses urine samples.
-
Sample stability for up to 7 days in AcornUPro-SEEK Urine Transport Reagent.
-
Turn-around time: 1-4 days from receipt of specimen.
The Evidence
The National Comprehensive Cancer Network (NCCN) Guidance recommends urinary molecular biomarker tests for patients to consider biopsies, in order to decrease unnecessary biopsies and increase the specificity of cancer detection, without missing a substantial number of higher-grade (Grade group ≥2) cancers.
European Association of Urology (EAU) Guidelines recommends the urinary molecular marker tests could help in discriminating between aggressive and non-aggressive tumours.
-
The AcornUPro-SEEK exhibits high sensitivity of 90%, facilitating accurate diagnosis of PCa, effectively for clinically significant prostate cancer.
-
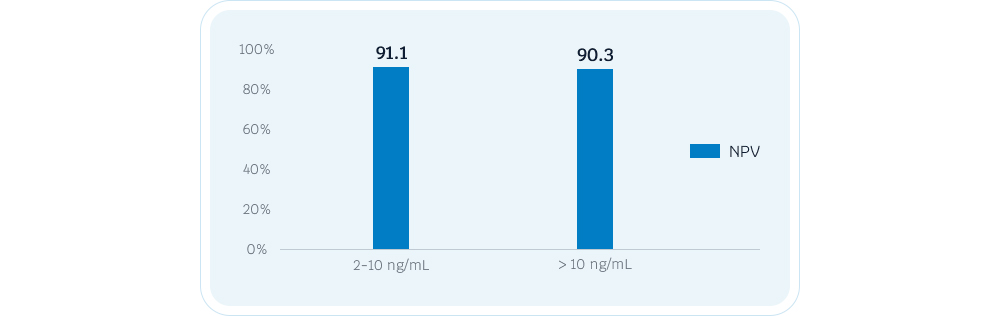
For patients in the PSA gray area (2-10ng/ml), AcornUPro-SEEK shows 91% of NPV, assisting in clinical diagnosis and reducing unnecessary biopsies.
AcornUPro-SEEK R&D model has has been granted “Breakthrough Devices Program” designation by the Food and Drug Administration (FDA).
The Suitable Individuals
- A man aged 50 or above.
- A man was older than 40 years with a family history of prostate cancer.
- Have a prostate-specific antigen (PSA) level between 2 and 10 ng/mL.
- Are considering a initial/repeat biopsy.
 Urothelial Carcinoma Products
Urothelial Carcinoma Products Prostate Cancer Product
Prostate Cancer Product Solid Tumor Product
Solid Tumor Product Hematologic Malignancy Product
Hematologic Malignancy Product
