The Clinical Use
- Provides information for clinically actionable genomic alterations and their associatedtargeted therapy, both approved and in clinical trials.
- Evaluates TMB and MSl to better inform immunotherapy decisions.
- Predicts efhcacy and toxicity of chemotherapy based on associated genetic biomarkers.
- Reveals potential resistance mechanism(s) to current therapies and options foralternative treatments.
- Assesses genetic predisposition to certain cancer types for early intervention.
- Information about the likely outcome of disease (your patients’ prognosis).
The Clinical Background
- National Comprehensive Cancer Network (NCCN®) guidance reflects more recent Food and Drug Administration (FDA) approvals of targeted therapies. They recommend testing solid tumor specimens for genomic variants and biomarkers that could potentially offer effective therapeutic options. Gene selection and product design are carried out in conjunction with authoritative journals such as NEJM and JCO.
- Many of these variants serve as predictive biomarkers for response to targeted therapies and immunotherapies. High TMB (TMB-H) and MSI (MSI-H) are predictive biomarkers of response to treatment with immune checkpoint inhibitors.
The Suitable Individuals
People with solid tumors whose diagnosis, prognosis, or treatment selection could be informed by the presence of genomic variants and MSI/TMB status.
The Solutions
The AcornOne808 analyzes genes primarily associated with onset conditions across major organ systems, including but not limited to lung, breast, gynecologic (ovarian, uterine/endometrial), gastrointestinal (colorectal, gastric, pancreatic), thyroid, genitourinary (renal/urinary tract, prostate), melanoma, and so on. Sequencing of over 800 genes, identified as relevant to cancer treatment, as well as testing of important immunotherapy biomarkers provides a comprehensive picture for the oncologist to formulate a treatment plan with their patients.
- CE-approved test
- Covering genomic regions of 808 genes
- Uses hybrid capture-based next-generation sequencing (NGS)
- Identifies the four main classes of genomic alterations (base substitutions, insertions and deletions, copy number alterations, and fusions
Biomarkers and companion diagnostic drugs
|
BIOMARKERS |
FDA-APPROVED THERAPY |
|
EGFR exon 19 deletions & EGFR exon 21 L858R alterations |
EGFR Tyrosine Kinase Inhibitors (TKI) |
|
EGFR exon 20 T790M alterations |
Tagrisso® (osimertinib) |
|
EGFR exon 20 insertion |
Rybrevant® (amivantamab-vmjw), Exkivity® (mobocertinib) |
|
ALK fusions |
Alecensa® (alectinib), Alunbrig® (brigatinib) Xalkori® (crizotinib), or Zykadia® (ceritinib), Lorbrena® (Lorlatinib) |
|
ROS1 fusions |
Xalkori® (Crizotinib), Rozlytrek® (entrectinib), Augtyro® (repotrectinib) |
|
RET fusions |
Retevmo® (Selpercatinib), Gavreto® (pralsetinib) |
|
MET single nucleotide variants (SNVs) and indels that lead to MET exon 14 skipping |
Tepmetko® (tepotinib), Tabrecta® (capmatinib) |
|
BRAF V600E |
Braftovi® (encorafenib) in combination with Mektovi® (binimetinib), Tafinlar® (dabrafenib) in combination with Mekinist® (trametinib) |
|
KRAS G12C |
Lumakras® (sotorasib), Krazati® (adagrasib) |
|
ERBB2 |
Enhertu® (trastuzumab deruxtecan) |
|
BIOMARKERS |
FDA-APPROVED THERAPY |
|
KRAS wild-type (absence of mutations in codons 12 and 13) |
Erbitux® (cetuximab) |
|
KRAS wild-type (absence of mutations in exons 2, 3, and 4) and NRAS wild type (absence of mutations in exons 2, 3, and 4) |
Vectibix® (panitumumab)
|
|
BRAF V600E |
Braftovi® (encorafenib) in combination with Erbitux® (cetuximab) |
|
BIOMARKERS |
FDA-APPROVED THERAPY |
|
BRCA1/2 |
Akeega® (niraparib/abiraterone acetate) in combination with Prednisone. Lynparza (Olaparib) in combination with abiraterone and prednisone or prednisolone. Rubraca® (Rucaparib) |
|
HRR (BRCA1, BRCA2, ATM, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, RAD51B, RAD51C, RAD51D, RAD54L) |
Lynparza® (Olaparib) |
|
HRR (ATM, ATR, BRCA1, BRCA2, CDK12, CHEK2, FANCA, MLH1, MRE11A, NBN, PALB2, RAD51C) |
Talzenna® (Talazoparib) |
|
BIOMARKERS |
FDA-APPROVED THERAPY |
|
FGFR3 |
Balversa® (Erdafitinib) |
|
BIOMARKERS |
FDA-APPROVED THERAPY |
|
MSI-High |
Keytruda® (Pembrolizumab) |
|
TMB-H(≥10 (mut/Mb) |
Keytruda® (Pembrolizumab) |
|
NTRK1/2/3 fusions |
Rozlytrek® (entrectinib), Vitrakvi® (Larotrectinib) |
|
RET fusions |
Retevmo® (Selpercatinib) |
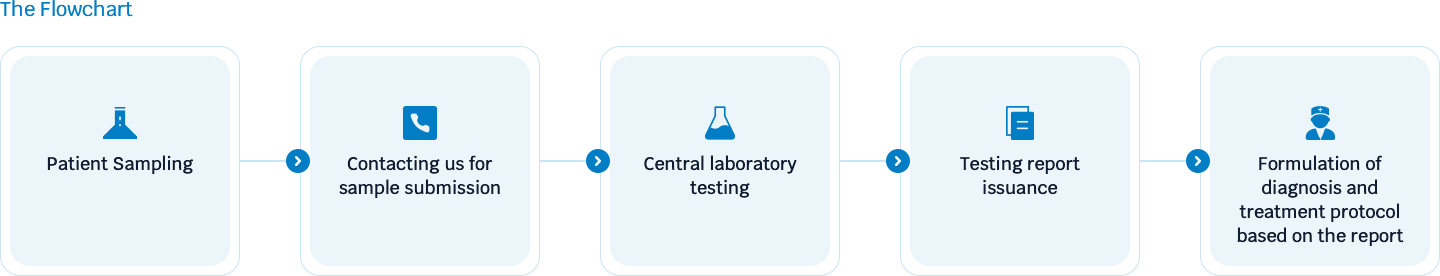
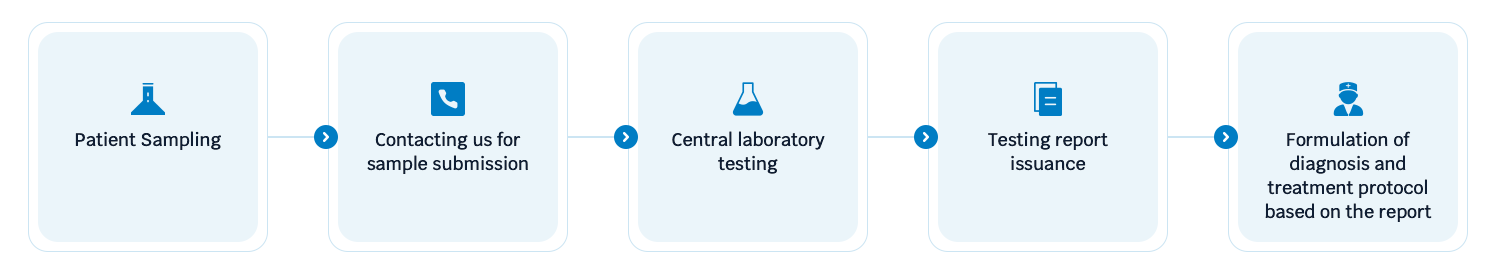
Flowchart
 Urothelial Carcinoma Products
Urothelial Carcinoma Products Prostate Cancer Product
Prostate Cancer Product Solid Tumor Product
Solid Tumor Product Hematologic Malignancy Product
Hematologic Malignancy Product
