The Need
Urothelial carcinoma (UC) includes bladder cancer (BC), renal pelvis cancer and ureteral cancer, of which 90% of UC is BC. BC is the 5th most common cancer in the western world. Blood in the urine (hematuria) is one of the most common signs of BC. Four out of five people with BC have blood in their urine, but 80–90% of patients with visible hematuria, and over 95% of patients with non-visible hematuria do NOT have cancer. In patients with hematuria, it can be difficult to decide which patients might have cancer and which patients might have a different condition.
Tests used in the detection or diagnosis of UC can include: Imaging tests, Urine cytology, Urinary molecular marker tests, Cystoscopy or Ureteroscopy.
- Imaging tests: Several types of imaging test can be used to visualize the inside of the body, such as ultrasound, CT scan, MRI scan, and X-ray, but small and flattened bladder tumors may also be difficult to visualize with imaging.
- Urine cytology: This test involves examining a urine sample under a microscope to determine if cancer or pre-cancer cells are visible. The Urine cytology has high sensitivity in HG and G3 tumours (84%), but low sensitivity in LG/G1 tumours (16%).
- Urinary molecular marker tests: There has been increasing use of molecular diagnostic tests to detect specific proteins or nucleic acids (RNA or DNA) in urine to diagnose cancer. The most commonly used methods are FISH and NMP22. Urine FISH has a sensitivity of 63% overall and 41% for low-grade cases, while NMP22 has a sensitivity of 58% overall and 25% for low-grade cases. Additionally, clinical factors like bladder inflammation and calculi affected FISH and NMP22.
- Cystoscopy or Ureteroscopy: Cystoscopy or Ureteroscopy enable the inside of the urethra and bladder to be examined and sampled. Cystoscopy is subjective, costly, highly dependent on the operator's expertise, and can be painful, often requiring anesthesia, which some patients may not tolerate. Ureteroscopy, on the other hand, carries the risk of damaging the ureter and potentially spreading the tumor.
The Solution
AcornUI-SEEK provides patients and clinicians with a simple, objective, and innovative urine test to detect UC. It demonstrates high sensitivity, facilitating accurate diagnosis of UC in patients presenting with hematuria, especially for patients with early UC. Additionally, high specificity to rule out UC, thus avoiding unnecessary cystoscopy or ureteroscopy.
- AcornUI-SEEK(The Early Detection Kit for Urothelial Carcinoma)has been approved by the NMPA in 2024.
-
The accuracy of AcornUI-SEEK test was validated in a clinical tria.
- Four DNA biomarkers (TERT/FGFR3 genes mutation and ONECUTE/VIM genes methylation) were intensively validated for the detection of UC.
- Quantitative Real-time PCR (qPCR) method makes the test economically and quickly.
- The non-invasive and painless test uses urine samples.
- Sample stability for up to 7 days in AcornUI-SEEK Urine Transport Reagent.
- Turn-around time: 1-4 days from receipt of specimen.
The Evidence
The 2024 Clinical Practice Guidelines for Urothelial Carcinoma of Chinese Society of Clinical Oncology (CSCO) recommends the detection of FGFR3 and TERT gene mutations, as well as methylation of the ONECUT2 and VM genes (AcornUI-SEEK) in urinary sediment for the detection of UC.
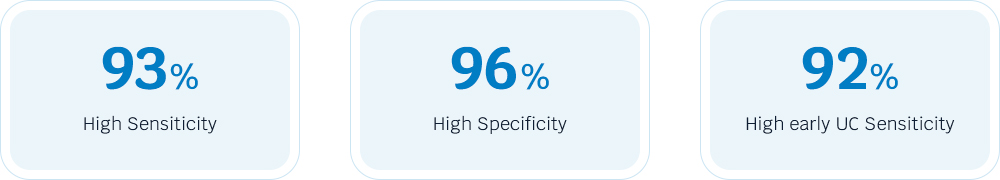
A study involving 1,080 patient urine samples demonstrated that AcornUI-SEEK had a sensitivity of 93% and specificity of 96% in patients with UC, showing excellent consistency with pathological diagnoses.
-
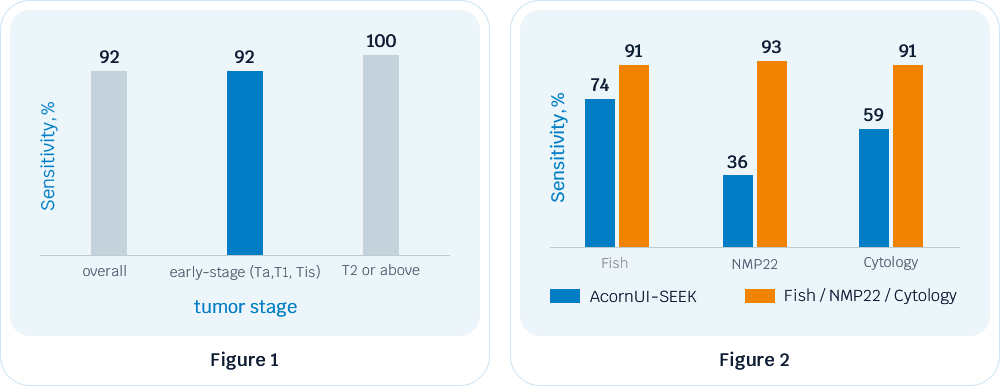
The test maintains stable detection rates of UC, with 92% in early-stage tumors and 100% in intermediate-advanced tumors (Figure 1).
-
It also shows higher sensitivity compared to cytology, FISH and NMP22 in detecting UC in patients with hematuria (Figure 2).
-
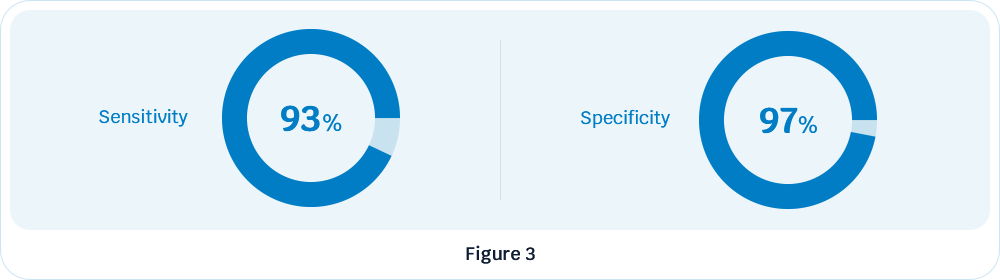
Even in patients with negative urine cytologic results, the AcornUI-SEEK test performed well, with a sensitivity of 93% and a specificity of 97% (Figure 3).
-
The results of the trial were published in Molecular Cancer in 2024.
AcornUI-SEEK R&D model has been granted "Breakthrough Devices Program" designation by the Food and Drug Administration (FDA).
The Suitable Individuals
- Screening of the population at risk of UC.
- Patients with haematuria or other symptoms suggestive of UC.
- Patients with negative urine cytology or unknown radiographic diagnosis need further testing.
- Patients who do not accept or are unsuitable for cystoscopy or ureteroscopy.
- Patients with inconclusive cystoscopy or ureteroscopy results require further diagnosis.
 Urothelial Carcinoma Products
Urothelial Carcinoma Products Prostate Cancer Product
Prostate Cancer Product Solid Tumor Product
Solid Tumor Product Hematologic Malignancy Product
Hematologic Malignancy Product